Two-Dimensional Infrared Spectroscopy
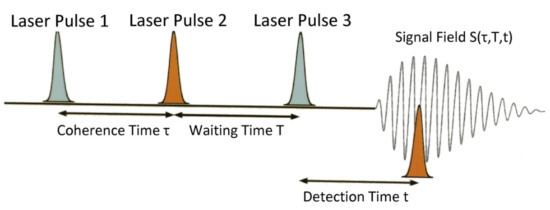
To gain higher spectral resolution, ultrafast dynamical information, and coupling mechanisms between individual transitions, we employ two-dimensional infrared spectroscopy (2D IR). 2D IR is based on a three-pulse excitation scheme using femtosecond IR laser excitation where the signal field S(τ,T,t) is measured with fixed waiting time T but varying τ and t. Fourier transforms along τ and t give a 2D IR spectrum. The detailed shapes of the diagonal and cross-peaks in the spectrum can then be used to study many different phenomena such as mode coupling, internal energy reorganization, and other relaxation properties of the distributions of states.
Temperature-Jump and Transient Infrared Spectroscopy
We are interested in observing some of the fastest events in folding. To this end, we apply laser induced temperture-jump and photochemical triggering to rapidly initiate folding/unfolding reactions, which we can monitor by probing various infrared absorption reporters that can measure either global or local dynamics. Combining a range of different such observables allows us to build mechanistic pictures of the folding "pathways" of some of the fastest folding proteins.
Fluorescence Correlation Spectroscopy and Single-Molecule FRET
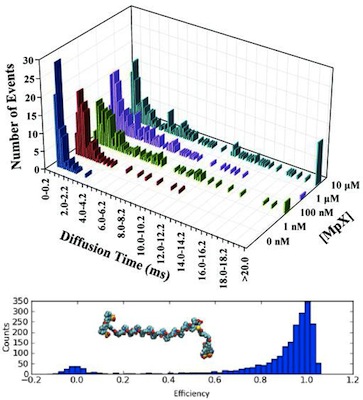
How do peptides interact with membranes? What are the size distributions and time scales associated with aggregate formation? Can we understand the structure and dynamics of intrinsicly dissordered peptides? These are all burning questions with the potential to answer pressing needs in the application of anti-microbials and amyloid-related diseases. Single molecule methods go a long way towards probing the micro-heterogeneity and dynamics associated with these processes.
Non-Natural Amino Acid IR and FRET Probes
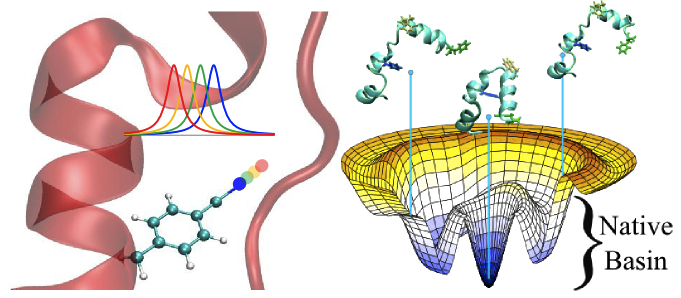
Design and application of new, small, IR and UV chromophores allows us to probe systems with minimal perturbation and high structural resolution.